SunStar Publishing Inc.
THE Food and Drug Administration has warned the public against the presence of four unregistered drug products in the market. In its Advisory 2024-1312 on Wednesday, October 2, 2024, the FDA said the public must avoid purchasing and consuming Norfloxacin Capsules 0.1 g, RHP Fusaimi Zhusheye – Furosemide Injection 2ml:20mg, HUAYI Vitamin C Injection 2ml:0.5g Ampoule, and Itraconazole Capsules 0.1g.
'FDA Post-Marketing Surveillance activities have verified that the abovementioned drug products have not gone through the registration process of the Agency, and have not been issued with proper authorization in the form of Certificate of Product Registration,' said the FDA. 'The FDA advises the public against the purchase and use of the following unregistered drug products,' it added.
FDA Unregistered Drugs Drug Products
Philippines Latest News, Philippines Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pope embarks on longest, farthest and most challenging trip to Asia, with China in the backgroundSunStar Publishing Inc.
Pope embarks on longest, farthest and most challenging trip to Asia, with China in the backgroundSunStar Publishing Inc.
Read more »
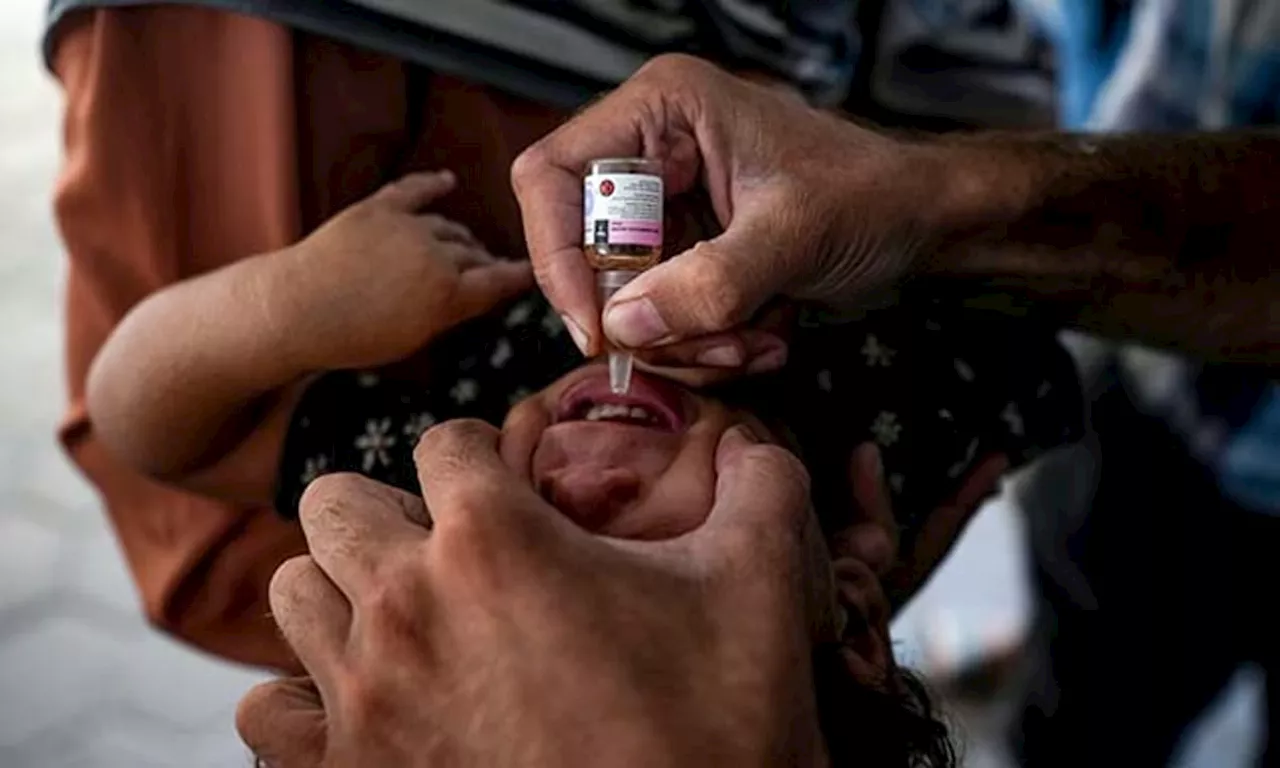 Large-scale polio vaccinations begin in war-ravaged Gaza after first case in 25 yearsSunStar Publishing Inc.
Large-scale polio vaccinations begin in war-ravaged Gaza after first case in 25 yearsSunStar Publishing Inc.
Read more »
 Marcos wants early dissemination of class, work suspension advisoriesSunStar Publishing Inc.
Marcos wants early dissemination of class, work suspension advisoriesSunStar Publishing Inc.
Read more »
 Some Lebanese who fear war is coming have an unusual backup plan: Moving to SyriaSunStar Publishing Inc.
Some Lebanese who fear war is coming have an unusual backup plan: Moving to SyriaSunStar Publishing Inc.
Read more »
 2 cops, lawyer dead in Tagaytay shootoutSunStar Publishing Inc.
2 cops, lawyer dead in Tagaytay shootoutSunStar Publishing Inc.
Read more »
 'Enteng' makes landfall in Casiguran, AuroraSunStar Publishing Inc.
'Enteng' makes landfall in Casiguran, AuroraSunStar Publishing Inc.
Read more »
